


Project Development
Project partners:
- Laboratory of the Biology and Pathology of the Eye, Charles University, Prague (CUNI)
- Department of Ophthalmology, University Hospital Kralovske Vinohrady, Prague (FNKV)
- Department of Ophthalmology, Oslo University Hospital, Oslo (OUS)
- Health Effects Laboratory, Norwegian Institute for Air Research, Kjeller (NILU)
The project consists of four work packages (WP):
- Reparation and potential self-regeneration of corneal endothelial cells (WP1 a WP2)
- Endothelial, limbal and conjunctival cells: organ culture and engineering of grafts (WP3)
- Testing DNA stability in cells from cornea and related tissues (WP4). Use of vitrification technique for long-term storage of cells and tissues.
Currently, experiments of all four WP are in progress:
WP1 and WP2:
The quality of the endothelium together with donor parameters directly determines the long-term surviving of the graft. To assess Descemet membrane endothelial keratoplasty with a stromal rim (DMEK-S), i.e. endothelial lamellar grafting technique, more than 200 corneas used for DMEK-S preparation were retrospectively evaluated, including donor data. (FNKV)
Paraffin sections from cultivated endothelium were prepared and their immunohistochemical analysis will be performed. (OUS, CUNI)
WP3:
1. Cultivation of the limbal stem cells:
Protocol optimization of the ex vivo isolation and cultivation of the limbal epithelial cells obtained from the corneoscleral rims is in progress (Fig. 1). We obtained 14 tissues for the cultivation on the plastic and decellularized amniotic membrane and in various types of media. The phenotype and stemness of cells are characterized by immunohistochemistry and fluorescence microscopy. Proliferation of the cultivated cells was tested by the WST-1 assay. (CUNI, FNKV)
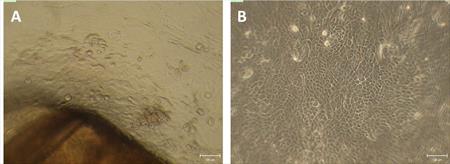
Fig. 1 Limbal stem cells obtained from corneoscleral rims (A) and after the cultivation on plastic (B)
The use of amniotic membrane as a carrier for cultivation of limbal cells is studied. We are exploring various systems and protocols of the deepithelialization of the amniotic membrane in order to determine a procedure with least resulting damage to epithelial cells and stromal surface. We tested Tryple select or dispase II dissociation, hypotonic or hypertonic solution for the decellularization process. The best results so far were obtained with Tryple select. (Fig.2) (CUNI)
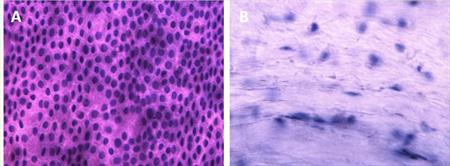
Fig. 2 Decellularization of the membrane is validated with the use of haemotoxilin and eosin staining
We prepare the epithelial cells from amniotic membrane for cultivation and intend to use these cells for enrichment of the cultivation environment with nutrients (Fig. 3) (CUNI)
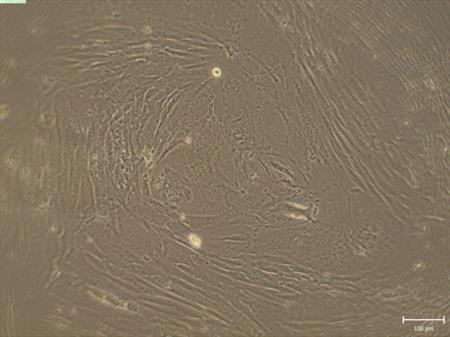
Fig. 3 Cultivation of the epithelial cells from the amniotic membrane
The storage of corneoscleral rims is evaluated under various cultivation conditions to find out the most suitable ones. We already obtained and prepared more than 50 tissues. (CUNI, FNKV)
The detection of corneal and conjunctival cytokeratins from the epithelium cultivated in two types of cultivation media and on two types of surfaces (plastic and amniotic membrane) was performed by immunohistochemistry (Fig. 4). The expression of the cytokeratins using PCR method is also performed. Publication summarizing these results is in preparation (OUS, CUNI)
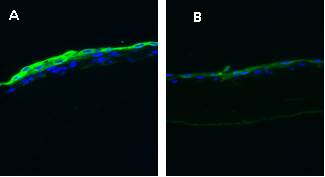
Fig. 4 Limbal stem cells cultivated form explants on amniotic membrane in media either with human serum (A) or in complex media (B) and stained for cytokeratin 13 by immunofluorescence.
The effect of trypsin dissociation on cultivated limbal stem cells is determined by Comet assay (Fig.5) (OUS)
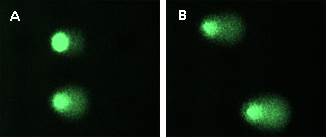
Fig.5 Comet assay – DNA damage analysis in corneal epithelium: 250 µl of trypsin (A) and 3 ml of trypsin (B).
2. Cultivation of goblet cells
Protocol optimization of goblet cell-rich culture is in progress. So far, conjunctival cells or tissues from more than 20 donors were used for cultivation. At first, we started with goblet cell cultivation from cell suspensions (Fig. 6A). Cell suspensions were prepared from the cells received from the surface of the eye by impression cytology or cytobrush technique. We tried different surfaces suitable for the cell culture (plastic, Matrigel, collagen, poly-L-lysine). Due to a low adhesion of the obtained cells, we started to try goblet cell cultivation from conjunctival explants. For this purpose we use conjunctiva obtained from lower fornix that is rich in conjunctival stem cells. So far, we successfully cultivated goblet cells from one donor (6B, C). Currently, cultivations from conjunctival explants are in progress and we search for the best composition of the media to support stem cell development into goblet cells. (CUNI)
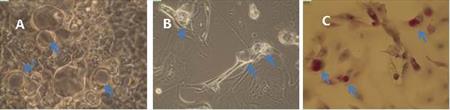
Fig. 6 Culture of goblet and epithelial cells from cell suspension (A), from conjunctival explant (B) and from conjunctival explant after histologic staining for presence of mucin inside the goblet cells (C).
3. Transplantations
The percentage of rejection episodes in patients after penetrating keratoplasty is retrospectively determined. By evaluation of more than 500 low and high risk patients we would like to determine the best storage technique for corneal tissue. (CUNI)
We finished our work about the influence of amniotic membrane transplantation on the expression of galectins in cornea in patients with keratitis acanthamoeba. Submitted paper is being reviewed. (CUNI)
WP4:
The specimens from patients suffering from melanoma were obtained by impression cytology and we assess genotoxical damage before and after iridium radiation therapy of the eye. We quantitatively evaluate the presence of micronuclei as a sign of genotoxical damage in epithelial cells of the conjunctiva (Fig. 7, 8). Preliminary results suggest that increase of number of micronuclei is time-limited and is related to the presence of the emitter. We demonstrated that micronucleus assay is convenient method for screening of genotoxical changes on the surface of the eye. (CUNI, NILU)
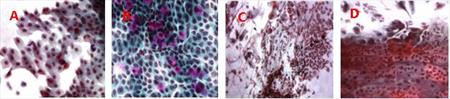
Fig. 7 Conjunctival epithelium before (A, B) and day 13 after (C, D) the irradiation.
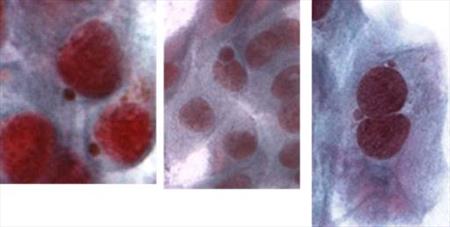
Fig. 8 Micronuclei present in the conjunctival epithelial cells after the iridium irradiation.
Assembly of vitrification system is in progress and we expect the device to be operational before the end of 2015. (CUNI)




